Fire and glow –
the black body
Contact
Deutsche Version
Fire and glow – | Dietrich Zawischa Contact Deutsche Version |
Flames shine if they contain particles of soot glowing and burning. This can be seen in the example of a candle's flame:
| In the lowest part of the flame there are no particles of soot, as there is abundant oxygen and the combustion of the vaporized wax is complete. There a weak bluish light can be seen which is emitted in the chemical reaction of combustion. Higher up in the flame there is less oxygen, and the remnants of partially burnt wax molecules agglutinate, forming soot particles emitting orange-coloured light in the heat of the flame. |
 |


Left: Candle flame mirrored in a black glass plate. Its colour can better be judged from the weaker mirror image, since the flame itself is over-exposed.
Right: Choosing the shutter speed such that the flame is not over-exposed, the surroundings are not seen any more. The colour of the flame is the same as that of its mirror image in the left picture.
(For these pictures, the white balance has been set to "Cloudy", see below. Settings: ISO 100, f:5.0. Left: 1/60 sec. Right: 1/1600 sec.)
Comparison with the next figure allows to estimate that the temperature in the brightest parts of the flame is almost 2000 Kelvin.
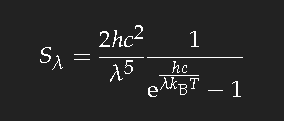
Back to the page “the origins of colour”