

Cuckoo Flower and Cornflower
| Dietrich Zawischa | Contact | Deutsche Version | |
What are the physical conditions to produce different colour stimuli? This question will be dealt with in this and the following sections.
Look at the colours in your environment: most objects with conspicuous colours are tinted or dyed. Textiles, for example: their natural colours are rather pale or even white; other colours are due to a treatment which deposits minute amounts of a substance, a dye, in the fibers. The white colour of a fabric has the same origin as that of snow or clouds: the single fibers consist of some colourless transparent substance. Light is reflected and refracted such that almost all of the light is scattered uniformly into all directions: This is perceived as "white".
If the tissue contains dye molecules, each fiber acts like a coloured filter, absorbing part of the light in some wavelength-region. Most of the light finally reaching the eye has passed one or more fibers, and correspondingly its spectral composition is different from that of the light source: the tissue is coloured.
White flowers do not contain any dye molecules (in particular, no "white dye", as there is no white dye); they are "snow-white" due to the many airfilled gaps in their tissue which cause multiple reflections and refractions. Very small concentrations of organic dyes result in vivid colours.
Thus we see that the colour of flowers, textiles and many other objects are due to the combination of two effects: the presence of dye molecules which selectively absorb light strongly already in very small concentrations, and secondly the multiple scattering by diffraction and reflection which would produce white in the absence of dyes.
In the two examples discussed, the multiple scattering was by air filled gaps or cavities. In an enamel varnish which must be hard-wearing and tough, the opacity is obtained by adding pigments.
Pigments consist of microscopically small grains which, however, are huge as compared to dye molecules. The grains of a white pigment are themselves colourless, but have a large refractive index and scatter light effectively even when embedded in resin or varnish, and thus produce the desired opacity. If the grains absorb light, then the pigment is coloured.
To understand the properties of dye molecules or pigment grains, we have to go back quite a bit. The most common colours can be explained only on the basis of quantum mechanics.
Everybody knows how a magnet attracts iron. In the vicinity of a magnet there are forces acting on other magnets, iron or some other substances: the magnet is surrounded by a field of force. Since ancient times, also electric forces are known; one notices that dust or light particles like paper snippets are attracted by pieces of amber which have been rubbed on wool or fur. Synthetic resin, ebonite and other materials exhibit this property as well.
Paper snippet attracted by amber
But only at the beginning of the 19th century, the connection between these two phenomena has been found, which started an industrial revolution.
Any time-dependent electric field induces a magnetic field, similar to an electric current. And a time-dependent magnetic field in turn induces an electric field. These findings got their quantitative formulation in the famous Maxwell equations.
As the magnetic field which is induced by an electric one is also time-dependent, it induces in turn a time-dependent electric field – and so on, thus electromagnetic waves may exist and propagate through space far from their sources.
In the vacuum, the velocity of electromagnetic waves is independent of their frequency, and is
c ≈ 3⋅108 m/sec = 300 000 km/sec,
per second more then seven times the earth's circumference.
We have no sense organs for electric and magnetic fields, but we can see light: electromagnetic waves with wavelengths between roughly 380 and 700 nm. Water and air absorb very little in this small interval while it is at the maximum of the sun's radiation.
From the speed of light c and the wavelength λ which can be measured using diffraction gratings, one obtains the frequency ν
| There are colour phenomena which can be fully understood if only the wave-nature of light is considered, e.g. the colours of soap bubbles (image to the right), of the rainbow, of iridescent birds' feathers, to mention only some of them. |
 |
There can be no doubt that light consists of electromagnetic waves. Diffraction experiments show that it behaves exactly as one expects from waves. Nevertheless, in the beginning of the 20th century, the old idea of light particles was revitalized.
It turned out that a wave of frequency ν, or, more general, any oscillating system with frequency ν, can absorb or give off energy only in small but finite "parcels", or quanta, which are proportional to the frequency:
Here ε is the energy quantum, the factor h is the famous Planck's constant. This constant h, when expressed in customary units is inconceivably small, so small that the quantisation can not be observed for low frequencies:
We therefore can never see any traces of quantisation in observing water waves, or any other processes which are accessible to our senses, and therefore the phenomena which occur at higher frequencies are strange to our minds, paradoxical, so to say. The paradox is known as "wave–particle dualism". Let us consider it in the case of the double-slit (or double-hole) experiment, which is discussed extensively in the sections on diffraction and interference in terms of light waves.
The two pictures below show the result of green light falling on a screen after having passed a diaphragm with one small hole in it (left) or with two small holes (right). If there is only one hole, the result is, roughly speaking, a diffuse light spot on the screen. If there are two holes of approximately equal size, this spot shows a pattern of stripes which result from interference between the waves coming from the two openings.

If the screen is replaced by photographic film, the intensity of the light source can be reduced (while the time of exposure is increased), so that the average time between absorptions of photons is so large that the presence of more than one photon at a time is highly improbable, so that the photons are dropping separately on the film.
One might think that the energy quanta would pass either the one or the other of the openings, and the interference pattern would vanish. But the pattern remains. Each black dot on the film results from a single photon, and the multitude of dots orders itself to produce the striped pattern. Thus, the probability to be absorbed by a grain of silver halide somewhere in the film to produce a black dot, is determined by the presence of both openings!
The situation is essentially the same in all cases of interference in the region of visible light or shorter wavelengths. Apart from few modern experiments with lasers, it is always so that each single photon interferes with itself. This means that the single photons are waves with considerable spatial extension; but when absorbed, all the energy is delivered at one point.
"The photon is born as a particle, lives as a wave, and dies as a particle"
This experiment and its interpretation shows one half of quantum mechanics, the fact that waves show properties of particles. The other half does not resolve this apparent contradiction: the fact that particles behave similarly, show properties of waves too.
Table salt, i.e. sodium chloride, or other sodium salts, brought into a flame, impart a characteristic orange-yellow colour to the flame. Other metals induce other colours (which is used for fireworks), and gas-discharge tubes emit coloured light, depending on the gas they contain. The deep red light of neon signs is well known.
Flame colours :
|
|
|
|
| ||||||||||||||||
| Lithium | Boron | Copper | Calcium |
An analysis of the light of a coloured flame shows the composition of the light as a mixture of different wavelengths, with few discrete wavelengths dominating.
Spectral "lines" occur, as in all apparatus which serve to analyze the light, the light has to pass a narrow slit. After that, by a prism or a diffraction grating, different wavelengths are deflected by different angles. An observer sees images of the slit at different angles, depending on the wavelength (colour), i.e. he sees "spectral lines".
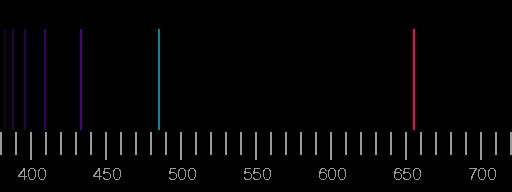
It is interesting and instructive to get (or to build) a simple spectroscope to observe the light of different lamps.
The appearance of discrete lines in the light emitted from atoms has been mysterious for a long time, until the mystery was resolved in the twenties of the 20th century.
The Bohr-Sommerfeld atomic model which may be considered as a precursor of the right explanation shall not be considered here; we will rather aim at an understanding of the correct quantum-mechanical phenomenon.
This now involves the "second half" of quantum mechanics, namely the fact that particles also behave like waves. The wave–particle dualism again, and again similar effects are never seen in macroscopic systems which are accessible to our immediate perception, so that our imagination fails. Quantum mechanics offers the possibility to understand things insofar as to predict the results of experiments and to relate complex situations to simpler ones; but to comprehend them intuitively is not possible. We may get acquainted with the strange phenomena, get accustomed to them, and accept the facts.
If a particle, say an electron, can behave as a wave, then diffraction phenomena should occur. A tiny double-slit should yield the same pattern of stripes which we encountered for light, and this should be observable in electron microscopy.
However, instead of double slits, crystal lattices offer more convenient diffraction gratings for electrons, e.g. in the form of thin metal foils.
From diffraction experiments one could determine the wavelength for electron beams, and the relation given first by L. de Broglie has been confirmed:
where p, the momentum of the particle is mass times velocity (as long as the velocity is small compared to the velocity of light). The constant h is the same as in equation (2).
For simplicity we consider the double-slit experiment for electrons. We know that the electron can not be divided into smaller subunits, but nevertheless, when passing the diaphragm, it must go through both slits like a wave. Otherwise there would be no interference pattern. As in the case of photons, the probability for a single electron to be absorbed at some spot of the photographic plate depends on the existence of both slits. If many electrons are absorbed, this probability distribution manifests itself in the interference pattern. We have seen that in the case of light we cannot say in advance where a quantum will be absorbed, we can only calculate probabilities – and now we see that this is exactly the case also for electrons.
The probability distribution shows the striped interference pattern, and therefore must be connected with some wave motion. The situation is analogous to the case of light. The probability to detect a light quantum is proportional to the light intensity which in turn is proportional to the square of the electric field strength (averaged over an oscillation cycle). This leads to the assumption that the probability to detect a point particle is also proportional to the squared absolute value of a wave-like quantity, the "wave function". This has been confirmed by the experimental data. Experimentally, the probability distribution can be obtained from series of measurements.
Writing for the wave function ψ(x,y,z,t), where x, y, z, and t are the coordinates of space and time, the probability to find the particle in a small volume ΔV is
Describing particle motion by time-dependent detection probabilities which can be obtained from a wave function, the concept of well defined trajectories has been abandoned. Instead, we arrive at the notion of "electron clouds" which describe the probability or rather density distribution of electrons bound in atoms or molecules.
| The simplest electron clouds are spherically symmetric. To the right a nonspherical example for the distribution of a single electron. |
| |
For macroscopic particles even of the size of a grain of dust the density distributions are almost always concentrated to such a small region of space, that the conception of trajectories is an excellent approximation.
In the interpretation of the mysterious wave function we have restricted ourselves to a minimum: it allows the prediction of results of experiments which can be performed at least in principle. The conception that a pointlike particle is moving in an unobservable way to produce the probability density distribution has been disproved: the motion of a charged particle such that a time-independent probability distribution results, would lead to electromagnetic radiation. No, the electrons behave rather as the photons do: as long as they are not observed (as pointlike particles), they are distributed over a larger spatial region, but when measured, one always gets the full electron charge or mass.
There is an important difference between electromagnetic waves / photons and matter waves / particles (electrons etc.): the photons are "gregarious", huge numbers can superpose themselves in identical waves, and this has the consequence that the waves become macroscopic and that we know electric and magnetic fields and electromagnetic waves. In contrast to this, electrons are "unsociable", loners, so to say: at most a second electron but with opposite spin direction can share the same spacial wave function. Therefore, matter waves are weird.
Particles of the gregarious kind are called bosons, the unsociable ones are fermions.
We will not deal with the theoretical considerations as the similarity between classical mechanics and geometric optics which in the beginning of the 20th century guided the discovery of quantum mechanics; also quantum mechanics shall not be explained in more detail here. It should only be said that the description of the states of particles or systems of particles by wave functions which may be obtained from the Schrödinger equation, is not the only one and not the most general possibility.
A first step towards the understanding of line spectra was the observation that the frequencies ν = c/λ may be ordered in a simple diagram. As the photon energies are proportional to the frequencies, it is quite plausible that it is more advantageous to consider the frequencies than the wavelengths. The energy of an emitted photon hν is just the difference of the atom's energies before and after its emission. Discrete photon energies are the consequence if atoms have discrete excitation energies. Thus it is possible to get from the spectrum information on the possible excited states of an atom, which may be represented in an energy level diagram or term scheme.
Atoms consist, as we know, of a positively charged nucleus containing almost all of the mass which is surrounded by electrons. In the beginning it was thought to be like a minute planetary system, with the electrons orbiting. But the electrical field produced by a moving electron is time-dependent, and electromagnetic waves should be radiated off. The permanent energy loss would lead to a collapse of the atom. Thus, the orbiting must not be understood too literally!
Let us consider now a hydrogen atom. A proton with one unit of charge binds an electron. If there are no external forces acting, the center of mass will be at rest or in uniform motion. The proton and the electron move around the center of mass. Because the nucleus is much heavier than the electron,
the center of mass is very close to the proton, and for simplicity we may assume that the nucleus is fixed and only the electron may move.
Thus the system is similar to a sun with one single planet, but it is so small that quantum mechanics is necessary to deal with it. Quantum mechanics tells us that we have to find a wave function for the electron. A function which is localized in the vicinity of the nucleus and vanishes at larger distances.
All waves, regardless of the medium which sustains them, have some general properties in common. We may try to learn something about localised waves looking at water waves. Waves on a lake or pond are not localized, but if the water is contained in a bowl, the waves cannot escape. We replace the attraction by the nucleus by a hard boundary. Although this model is very crude, we may expect some similar features.
 |
 |
 |
 |
| (a) | (b) | (c) | (d) |
Whereas in an extended water surface (pond, lake, or sea) the wavelengths are not restricted, and therefore also arbitrary frequencies are possible, the standing waves shown in the above figures have fixed frequencies (here depending on the size of the bowl, the density of the fluid, gravity and surface tension). It is a general feature of waves in finite systems that they are superpositions of normal modes with fixed frequencies. Organ pipes and vibrating strings, in fact almost all musical instruments may serve as examples. The finite wave functions of the hydrogen atom's electron therefore can also oscillate only with certain eigenfrequencies, and, because of the connection between energy and frequency, E = hν (equation 2), discrete energy levels are the result.
As every state of the electron must be describable by a wave function, and as there is a standing wave with lowest frequency, the collapse of the atom is not possible. (If the water in the bowl is at rest without any wave this corresponds to the case where there is no electron at all: the nucleus and vacuum.)
Neon which emits red light in gas discharge tubes, is a colourless gas, in spite of the fact that it also can absorb light of the same wavelength. However, the absorption occurs only in such a narrow wavelength-interval and with little probability that it is imperceptible.
|
Molecules of organic dyes can absorb light in a much broader region of wavelengths, and the absorption probability is much higher. Both properties are related to the size of the molecules, as will be discussed in more deteil in a special section. Emission and absorption of electromagnetic waves is just the same as what is done by antennas in wireless communication. Consider a VHF radio tuned to 100 MHz: it can absorb energy from electromagnetic waves with 3 m wavelength. If we provide this radio with an antenna which is only 1 mm long, the reception will be very insatisfactory, as, due to the smallness of the antenna, only little energy will be absorbed. If we take a larger antenna of, say 2 cm length, the situation is significantly improved. |  Flower of corn poppy (Papaver rhoeas L.) |
We know that for a good transmission, i.e. absorption of energy by the receiver, the length of the antenna should be of the same order of magnitude as the wavelength. The same is true for the radiating power of a transmitting aerial. Now, the sizes of atoms and molecules correspond roughly to the lengths of antennas. But while light has wavelengths around 500 nm, the atomic sizes are of the order of 0.1 nm!
Dye molecules are like small strings or loops, along which the electrons can move freely like in a thin wire. They are effectively small antennas. Though they are small compared to the wavelengths of light, the adjustment is much better than that of atoms, and correspondingly they absorb much better than atoms or small molecules.
The other important property of larger molecules is that they have many vibrational degrees of freedom. Absorbed energy may lead to vibrations and may be given off easily as heat. Therefore, the molecules are not as selective as atoms with respect to absorbable energy: if a photon has too much, the surplus can be given off as heat, if it has too little energy, thermal motion may contribute. This greatly increases the band width for absorption.
Back to the page “the origins of colour”
Continue with: Atomic spectra